16.2 Psychopharmacology and Brain-Based Therapies
Learning Objectives
- Describe how the majority of psychoactive drugs work in the brain.
- Describe the most common drugs used in the treatment of disorders.
- Describe why grapefruit is dangerous to consume with many psychotropic medications.
- Explain why there is controversy regarding pharmacotherapy for children and adolescents.
Virtually any drug that changes the way you feel does this by altering how neurons communicate with each other. Neurons, more than 100 billion in your nervous system, communicate with each other by releasing a chemical (i.e., neurotransmitter) across a tiny space between two neurons (i.e., synapse). When the neurotransmitter crosses the synapse, it binds to a postsynaptic receptor (i.e. protein) on the receiving neuron, and the message may then be transmitted onward. Obviously, neurotransmission is far more complicated than this, but the first step is understanding that virtually all psychoactive drugs interfere with or alter how neurons communicate with each other.
There are many neurotransmitters. Some of the most important in terms of psychopharmacological treatment and drugs of abuse are outlined in the table below. The neurons that release these neurotransmitters, for the most part, are localized within specific circuits of the brain that mediate these behaviours.
| Neurotransmitter | Abbreviation | Behaviour or Diseases Related |
| Acetylcholine | ACh | Learning and memory; Alzheimer’s disease muscle movement in the peripheral nervous system |
| Dopamine | DA | Reward circuits; motor circuits involved in Parkinson’s disease; schizophrenia |
| Norepinephrine | NE | Arousal; depression |
| Serotonin | 5HT | Depression; aggression; schizophrenia |
| Glutamate | GLU | Learning; major excitatory neurotransmitter in the brain |
| Gamma-Aminobutyric Acid | GABA | Anxiety disorders; epilepsy; major inhibitory neurotransmitter in the brain |
| Endogenous Opioids | Endorphins, Enkephalins | Pain; analgesia; reward |
Psychoactive drugs can either increase activity at the synapse (i.e., agonists) or reduce activity at the synapse (i.e., antagonists). Different drugs do this by different mechanisms, and some examples of agonists and antagonists are presented in the table below. For each example, the drug’s trade name, which is the name of the drug provided by the drug company, and generic name are provided.
| Drug | Mechanism | Use | Agonist/Antagonist |
| L-dopa | Increase synthesis of DA | Parkinson’s disease | Agonist for DA |
| Adderall (mixed salts amphetamine) | Increase release of DA, NE | ADHD | Agonist for DA, NE |
| Ritalin (methylphenidate) | Blocks removal of DA, NE, and lesser (5HT) from synapse | ADHD | Agonist for DA, NE mostly |
| Aricept (donepezil) | Blocks removal of ACh from synapse | Alzheimer’s disease | Agonist for ACh |
| Prozac (flouxetine) | Blocks removal of 5HT from synapse | Depression, obsessive-compulsive disorder | Agonist for 5HT |
| Seroquel (quetiapine) | Blocks DA and 5HT receptors | Schizophrenia, bipolar disorder | Antagonist for DA, 5HT |
| Revia (naltrexone) | Blocks opioid post-synaptic receptors | Alcoholism, opioid addiction | Antagonist for opioids |
It is very important to realize that drugs also have effects on other neurotransmitters. This contributes to the kinds of side effects that are observed when someone takes a particular drug. The reality is that no drugs currently available work only exactly where we would like in the brain or only on a specific neurotransmitter. In many cases, individuals are prescribed one psychotropic drug — a drug that changes mood or emotion, usually drugs prescribed for various mental conditions, such as depression, anxiety, or schizophrenia — but then may also have to take additional drugs to reduce the side effects caused by the initial drug. Sometimes individuals stop taking medication because the side effects can be so profound. The table below shows some common medications for various disorders, along with their side effects.
| Type of Medication | Used to Treat | Brand Names of Commonly Prescribed Medications | How They Work | Side Effects |
|---|---|---|---|---|
| Antipsychotics (developed in the 1950s) | Schizophrenia and other types of severe thought disorders | Haldol, Mellaril, Prolixin, Thorazine | Treat positive psychotic symptoms such as auditory and visual hallucinations, delusions, and paranoia by blocking the neurotransmitter dopamine | Long-term use can lead to tardive dyskinesia, involuntary movements of the arms, legs, tongue and facial muscles, resulting in Parkinson’s-like tremors |
| Atypical Antipsychotics (developed in the late 1980s) | Schizophrenia and other types of severe thought disorders | Abilify, Risperdal, Clozaril | Treat the negative symptoms of schizophrenia, such as withdrawal and apathy, by targeting both dopamine and serotonin receptors; newer medications may treat both positive and negative symptoms | Can increase the risk of obesity and diabetes as well as elevate cholesterol levels; constipation, dry mouth, blurred vision, drowsiness, and dizziness |
| Anti-Depressants | Depression and increasingly for anxiety | Paxil, Prozac, Zoloft (selective serotonin reuptake inhibitors); Tofranil and Elavil (tricyclics) | Alter levels of neurotransmitters such as serotonin and norepinephrine | Selective serotonin reuptake inhibitors: headache, nausea, weight gain, drowsiness, reduced sex drive Tricyclics: dry mouth, constipation, blurred vision, drowsiness, reduced sex drive, increased risk of suicide |
| Anti-Anxiety Agents | Anxiety and agitation that occur in OCD, PTSD, panic disorder, and social phobia | Xanax, Valium, Ativan | Depress central nervous system activity | Drowsiness, dizziness, headache, fatigue, lightheadedness |
| Mood Stabilizers | Bipolar disorder | Lithium, Depakote, Lamictal, Tegretol | Treat episodes of mania as well as depression | Excessive thirst, irregular heartbeat, itching or rash, swelling (face, mouth, and extremities), nausea, loss of appetite |
| Stimulants | ADHD | Adderall, Ritalin | Improve ability to focus on a task and maintain attention | Decreased appetite, difficulty sleeping, stomachache, headache |
| Data source: Spielman et al., 2019. | ||||
Using stimulants to treat ADHD
Attention-deficit/hyperactivity disorder (ADHD) is frequently treated with biomedical therapy, usually along with cognitive behavioural therapy (CBT). The most commonly prescribed drugs for ADHD are psychostimulants, including Ritalin, Adderall, and Dexedrine. Short-acting forms of the drugs are taken as pills and last between four to 12 hours, but some of the drugs are also available in long-acting forms, such as skin patches that can be worn on the hip and last up to 12 hours. In most cases, the patch is applied early in the morning and worn all day.
Stimulants improve the major symptoms of ADHD, including inattention, impulsivity, and hyperactivity, often dramatically, in about 75% of the children who take them (Greenhill, Halperin, & Abikof, 1999), but the effects of the drugs wear off quickly. Additionally, the best drug and best dosage varies from person to person, so it may take some time to find the correct combination.
It may seem surprising to you that a disorder that involves hyperactivity is treated with a psychostimulant, a drug that normally increases activity. The answer lies in the dosage. When large doses of stimulants are taken, they increase activity, but in smaller doses, the same stimulants improve attention and decrease motor activity (Zahn, Rapoport, & Thompson, 1980).
The most common side effects of psychostimulants in children include decreased appetite, weight loss, sleeping problems, and irritability as the effect of the medication tapers off. Stimulant medications may also be associated with a slightly reduced growth rate in children, although in most cases growth is not permanently affected (Spencer, Biederman, Harding, & O’Donnell, 1996).
Antidepressant medications
Antidepressant medications are drugs designed to improve moods. Although they are used primarily in the treatment of depression, they are also effective for patients who suffer from anxiety, phobias, and obsessive-compulsive disorders. Antidepressants work by influencing the production and reuptake of neurotransmitters that relate to emotion, including serotonin, norepinephrine, and dopamine. Although exactly why they work is not yet known, as the amount of the neurotransmitters in the central nervous system (CNS) is increased through the action of the drugs, the person often experiences less depression.
The original antidepressants were the tricyclic antidepressants, with the brand names of Tofranil and Elavil, and the monamine oxidase inhibitors (MAOIs). These medications work by increasing the amount of serotonin, norepinephrine, and dopamine at the synapses, but they also have severe side effects including potential increases in blood pressure and the need to follow particular diets.
The antidepressants most prescribed today are the selective serotonin reuptake inhibitors (SSRIs), including Prozac, Paxil, and Zoloft, which are designed to selectively block the reuptake of serotonin at the synapse, thereby leaving more serotonin available in the CNS. SSRIs are safer and have fewer side effects than the tricyclics or the MAOIs (Fraser, 2000; Hollon, Thase, & Markowitz, 2002). SSRIs are effective, but patients taking them often suffer a variety of sometimes unpleasant side effects, including dry mouth, constipation, blurred vision, headache, agitation, drowsiness, as well as a reduction in sexual enjoyment.
There has been concern that SSRIs may increase the risk of suicide among teens and young adults, probably because when the medications begin working they give patients more energy, which may lead them to commit the suicide that they had been planning but lacked the energy to go through with (Barbui, Esposito, & Cipriani, 2009). This concern has led doctors to be more selective about prescribing antidepressants to this age group (Healy & Whitaker, 2003; Simon, 2006; Simon, Savarino, Operskalski, & Wang, 2006).
Because the effects of antidepressants may take weeks or even months to develop, doctors usually work with each patient to determine which medications are most effective and may frequently change medications over the course of therapy. In some cases, other types of antidepressants may be used instead of, or in addition to, the SSRIs. These medications also work by blocking the reuptake of neurotransmitters, including serotonin, norepinephrine, and dopamine. Brand names of these medications include Effexor and Wellbutrin.
Patients who are suffering from bipolar disorder are not helped by the SSRIs or other antidepressants because their disorder also involves the experience of overly positive moods. Treatment is more complicated for these patients, often involving a combination of antipsychotics and antidepressants along with mood stabilizing medications (McElroy & Keck, 2000). The most well-known mood stabilizer, lithium carbonate, simply referred to as lithium, is used widely to treat mania associated with bipolar disorder. Available in Canada for more than 60 years, the medication is used to treat acute manic episodes and as a long-term therapy to reduce their frequency and severity. Anticonvulsant medications can also be used as mood stabilizers. Another drug, Depakote, has also proven very effective, and some bipolar patients may do better with it than with lithium (Kowatch et al., 2000).
People who take lithium must have regular blood tests to be sure that the levels of the drug are in the appropriate range. Potential negative side effects of lithium are loss of coordination, slurred speech, frequent urination, and excessive thirst. Though side effects often cause patients to stop taking their medication, it is important that treatment be continuous, rather than intermittent. Recently, Health Canada updated safety information and treatment recommendations for lithium after finding that taking lithium carries a risk of high blood calcium, or hypercalcemia, and is sometimes associated with a hormone disorder known as hyperparathyroidism (Canadian Press, 2014). There is no cure for bipolar disorder, but drug therapy does help many people.
Antianxiety medications
Antianxiety medications are drugs that help relieve fear or anxiety. They work by increasing the action of the neurotransmitter GABA. The increased level of GABA helps inhibit the action of the sympathetic division of the autonomic nervous system, creating a calming experience.
The most common class of antianxiety medications is the tranquilizers, known as benzodiazepines. These drugs, which are prescribed millions of times a year, include Ativan, Valium, and Xanax. The benzodiazepines act within a few minutes to treat mild anxiety disorders but also have major side effects. They are addictive, frequently leading to tolerance, and they can cause drowsiness, dizziness, and unpleasant withdrawal symptoms including relapses into increased anxiety (Otto et al., 1993). Furthermore, because the effects of the benzodiazepines are very similar to those of alcohol, they are very dangerous when combined with it.
Antipsychotic medications
Until the middle of the 20th century, schizophrenia was inevitably accompanied by the presence of positive symptoms, including bizarre, disruptive, and potentially dangerous behaviour. As a result, schizophrenics were locked in asylums to protect them from themselves and to protect society from them. In the 1950s, a drug called chlorpromazine (e.g., Thorazine) was discovered that could reduce many of the positive symptoms of schizophrenia. Chlorpromazine was the first of many antipsychotic drugs.
Antipsychotic drugs, known as neuroleptics, are drugs that treat the symptoms of schizophrenia and related psychotic disorders. Today, there are many antipsychotics, including Thorazine, Haldol, Clozaril, Risperdal, and Zyprexa. Some of these drugs treat the positive symptoms of schizophrenia, and some treat the positive, negative, and cognitive symptoms.
The discovery of chlorpromazine and its use in clinics has been described as the single greatest advance in psychiatric care because it has dramatically improved the prognosis of patients in psychiatric hospitals worldwide. Using antipsychotic medications has allowed hundreds of thousands of people to move out of asylums into individual households or community mental health centres, and in many cases, it has allowed sufferers to live near-normal lives.
Antipsychotics reduce the positive symptoms of schizophrenia by reducing the transmission of dopamine at the synapses in the limbic system, and they improve negative symptoms by influencing levels of serotonin (Marangell, Silver, Goff, & Yudofsky, 2003). Despite their effectiveness, antipsychotics have some negative side effects, including restlessness, muscle spasms, dizziness, and blurred vision. In addition, their long-term use can cause permanent neurological damage, a condition called tardive dyskinesia that causes uncontrollable muscle movements, usually in the mouth area (National Institute of Mental Health, 2008). Newer antipsychotics treat more symptoms with fewer side effects than older medications (Casey, 1996).
Direct brain intervention therapies
In cases of severe disorder, it may be desirable to directly influence brain activity through electrical activation of the brain or through brain surgery. Electroconvulsive therapy (ECT) is a medical procedure designed to alleviate psychological disorder in which electric currents are passed through the brain, deliberately triggering a brief seizure (see Figure 16.7). ECT has been used since the 1930s to treat severe depression.
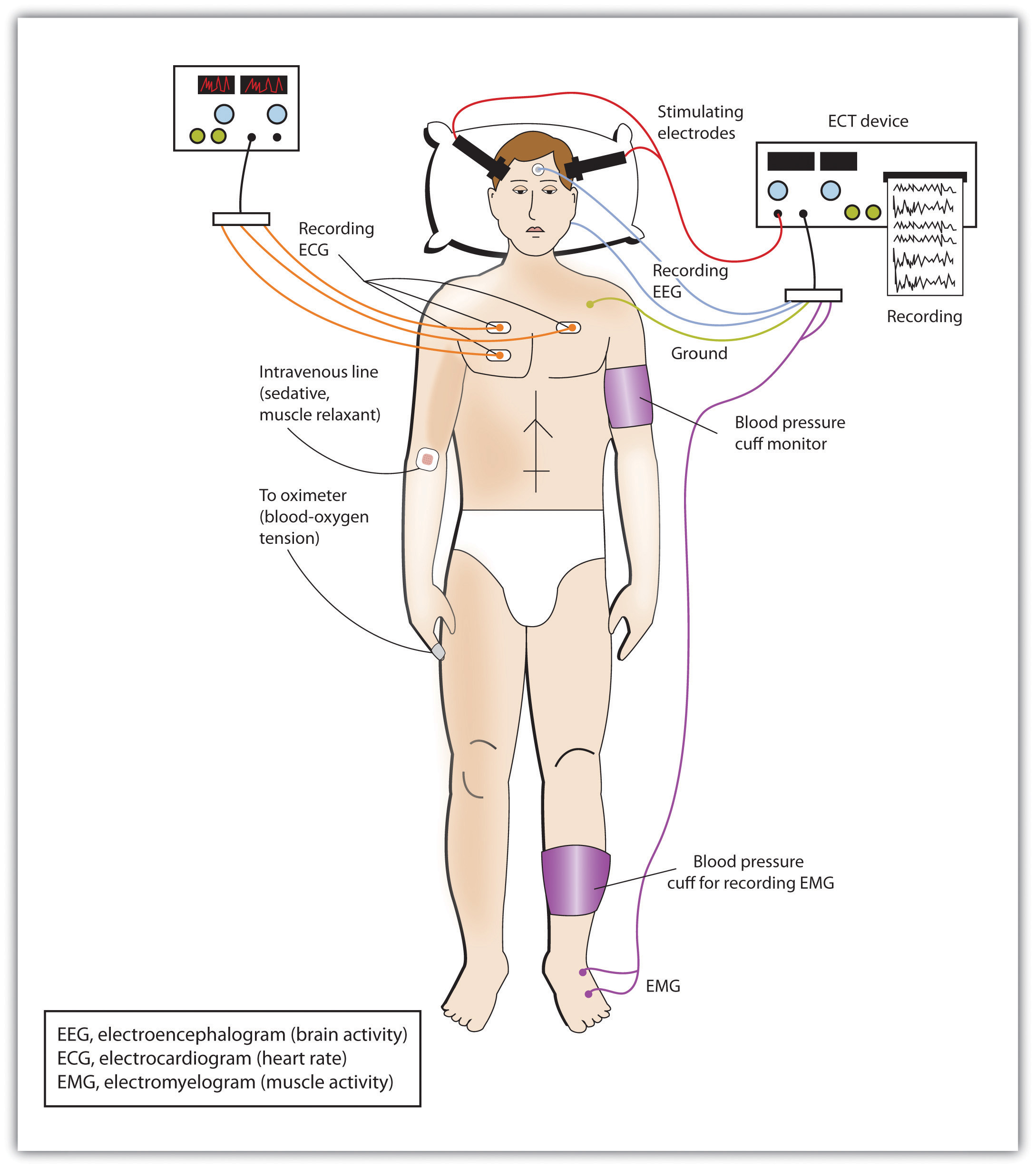
When it was first developed, the procedure involved strapping the patient to a table before the electricity was administered. The patient was knocked out by the shock, went into severe convulsions, and awoke later, usually without any memory of what had happened. Today, ECT is used only in the most severe cases when all other treatments have failed, and the practice is more humane. The patient is first given muscle relaxants and a general anesthesia, and precisely calculated electrical currents are used to achieve the most benefit with the fewest possible risks.
ECT is very effective. About 80% of people who undergo three sessions of ECT report dramatic relief from their depression. ECT reduces suicidal thoughts and is assumed to have prevented many suicides (Kellner et al., 2005). On the other hand, the positive effects of ECT do not always last; over one-half of patients who undergo ECT experience relapse within one year, although antidepressant medication can help reduce this outcome (Sackheim et al., 2001). ECT may also cause short-term memory loss or cognitive impairment (Abrams, 1997; Sackheim et al., 2007).
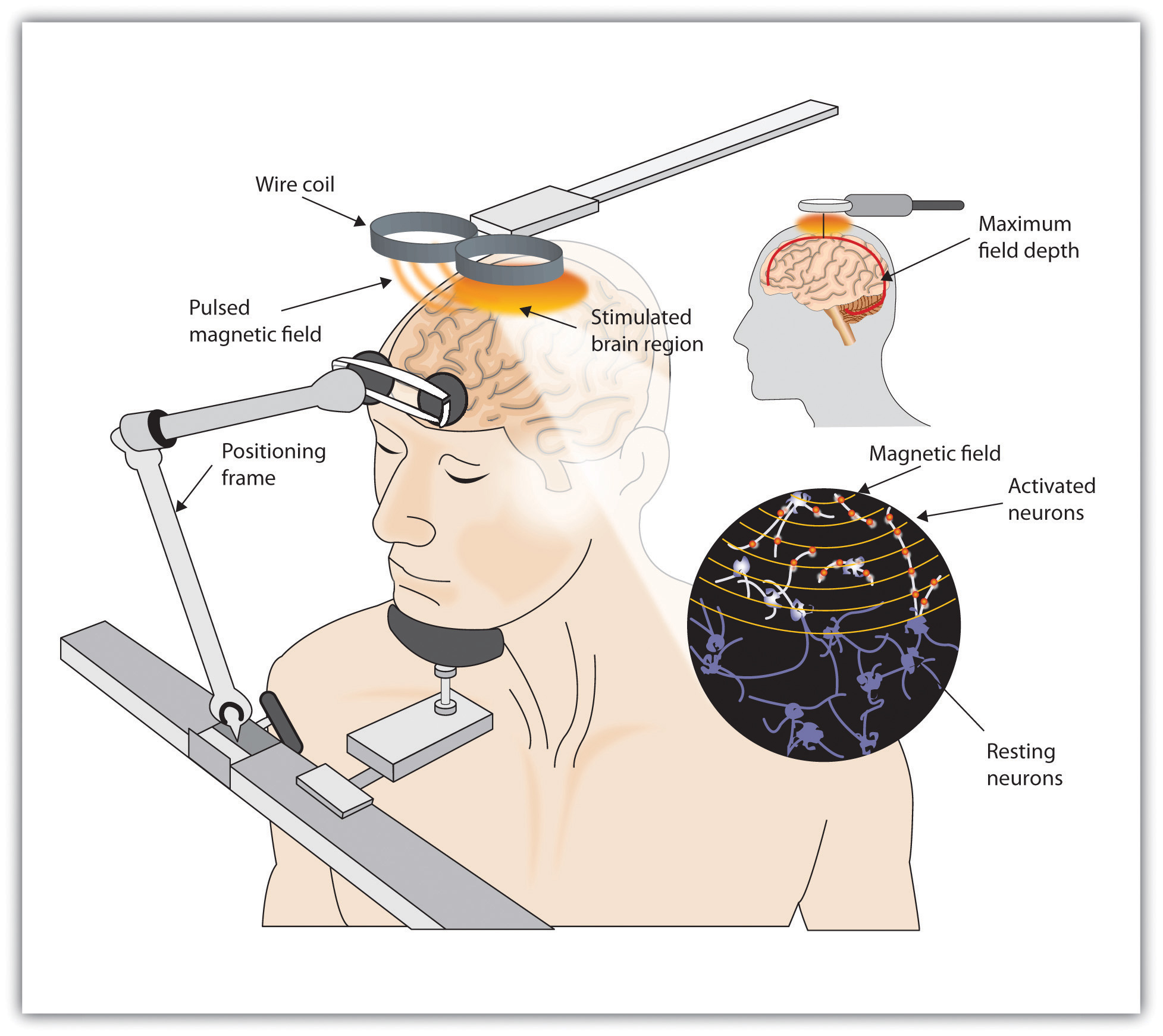
Although ECT continues to be used, newer approaches to treating chronic depression are also being developed. A newer and gentler method of brain stimulation is transcranial magnetic stimulation (TMS), which is a medical procedure designed to reduce psychological disorder that uses a pulsing magnetic coil to electrically stimulate the brain (see Figure 16.8). TMS seems to work by activating neural circuits in the prefrontal cortex, which is less active in people with depression, causing an elevation of mood. TMS can be performed without sedation, does not cause seizures or memory loss, and may be as effective as ECT (Loo, Schweitzer, & Pratt, 2006; Rado, Dowd, & Janicak, 2008). TMS has also been used in the treatment of Parkinson’s disease and schizophrenia.
Still other biomedical therapies are being developed for people with severe depression that persists over years. One approach involves implanting a device in the chest that stimulates the vagus nerve, a major nerve that descends from the brain stem toward the heart (Corcoran, Thomas, Phillips, & O’Keane, 2006; Nemeroff et al., 2006). When the vagus nerve is stimulated by the device, it activates brain structures that are less active in severely depressed people.
Psychosurgery, that is, surgery that removes or destroys brain tissue in the hope of improving disorder, is reserved for the most severe cases. The most well-known psychosurgery is the prefrontal lobotomy. Developed in 1935 by Nobel Prize winner Egas Moniz to treat severe phobias and anxiety, the procedure destroys the connections between the prefrontal cortex and the rest of the brain. Lobotomies were performed on thousands of patients. The procedure, which was never validated scientifically, left many patients in worse condition than before, subjecting the already suffering patients and their families to further heartbreak (Valenstein, 1986). Perhaps the most notable failure was the lobotomy performed on Rosemary Kennedy, the sister of U.S. President John F. Kennedy, which left her severely incapacitated.
There are very few centres that still conduct psychosurgery today, and when such surgeries are performed, they are much more limited in nature and called cingulotomy (Dougherty et al., 2002). The ability to more accurately image and localize brain structures using modern neuroimaging techniques suggests that new, more accurate, and more beneficial developments in psychosurgery may soon be available (Sachdev & Chen, 2009).
Pharmacokinetics: What it is, and why it is important
While this section may sound more like pharmacology, it is important to realize how important pharmacokinetics can be when considering psychoactive drugs. Pharmacokinetics refers to how the body handles a drug that we take. As mentioned earlier, psychoactive drugs exert their effects on behaviour by altering neuronal communication in the brain, and the majority of drugs reach the brain by travelling in the blood. The acronym ADME is often used, which stands for absorption (i.e., how the drug gets into the blood), distribution (i.e., how the drug gets to the organ of interest — in this case, the brain), metabolism (i.e., how the drug is broken down so it no longer exerts its psychoactive effects), and excretion (i.e., how the drug leaves the body). We will talk about a couple of these to show their importance for considering psychoactive drugs.
Drug administration
There are many ways to take drugs, and these routes of drug administration can have a significant impact on how quickly that drug reaches brain. The most common route of administration is oral administration, which is relatively slow and — perhaps surprisingly — often the most variable and complex route of administration. Drugs enter the stomach and then get absorbed by the blood supply and capillaries that line the small intestine. The rate of absorption can be affected by a variety of factors, including the quantity and the type of food in the stomach (e.g., fats versus proteins). This is why the medicine label for some drugs (e.g., antibiotics) may specifically state foods that you should or should not consume within an hour of taking the drug because they can affect the rate of absorption. Two of the most rapid routes of administration include inhalation (e.g., smoking or gaseous anesthesia) and intravenous (IV) in which the drug is injected directly into the vein and, hence, the blood supply. Both of these routes of administration can get the drug to the brain in less than 10 seconds. IV administration also has the distinction of being the most dangerous because if there is an adverse drug reaction, there is very little time to administer any antidote, as in the case of an IV heroin overdose.
Why might the speed in which a drug gets to the brain be important? If a drug activates the reward circuits in the brain and it reaches the brain very quickly, the drug has a high risk for abuse and addiction. Psychostimulants like amphetamine or cocaine are examples of drugs that have high risk for abuse because they are agonists at DA neurons involved in reward and because these drugs exist in forms that can be either smoked or injected intravenously. Some argue that cigarette smoking is one of the hardest addictions to quit, and although part of the reason for this may be that smoking gets the nicotine into the brain very quickly and indirectly acts on DA neurons, it is a more complicated story. For drugs that reach the brain very quickly, not only is the drug very addictive, but so are the cues associated with the drug (Rohsenow, Niaura, Childress, Abrams, & Monti, 1990). For a “crack” cocaine user, this could be the pipe that they use to smoke the drug. For a cigarette smoker, however, it could be something as normal as finishing dinner or waking up in the morning, if that is when the smoker usually has a cigarette. For both the crack user and the cigarette smoker, the cues associated with the drug may actually cause craving that is alleviated by lighting a cigarette or using crack (i.e., relapse). This is one of the reasons individuals that enroll in drug treatment programs, especially out-of-town programs, are at significant risk of relapse if they later find themselves in proximity to old haunts, friends, and so on. However, this is much more difficult for a cigarette smoker. How can someone avoid eating or avoid waking up in the morning? These examples help you begin to understand how important the route of administration can be for psychoactive drugs.
Drug metabolism
Metabolism involves the breakdown of psychoactive drugs, and this occurs primarily in the liver. The liver produces enzymes, which are proteins that speed up a chemical reaction, and these enzymes help catalyze a chemical reaction that breaks down psychoactive drugs. Enzymes exist in “families,” and many psychoactive drugs are broken down by the same family of enzymes, the cytochrome P450 superfamily. There is not a unique enzyme for each drug; rather, certain enzymes can break down a wide variety of drugs. Tolerance to the effects of many drugs can occur with repeated exposure; that is, the drug produces less of an effect over time, so more of the drug is needed to get the same effect. This is particularly true for sedative drugs like alcohol or opiate-based painkillers. Metabolic tolerance is one kind of tolerance, and it takes place in the liver. Some drugs (e.g., alcohol) cause enzyme induction, which is an increase in the enzymes produced by the liver. For example, chronic drinking results in alcohol being broken down more quickly, so the alcoholic needs to drink more to get the same effect — of course, until so much alcohol is consumed that it damages the liver, since alcohol can cause fatty liver or cirrhosis.
Recent issues related to psychotropic drugs and metabolism
Certain types of food in the stomach can alter the rate of drug absorption, and other foods can also alter the rate of drug metabolism. The most well known is grapefruit juice. Grapefruit juice suppresses cytochrome P450 enzymes in the liver, and these liver enzymes normally break down a large variety of drugs, including some of the psychotropic drugs. If the enzymes are suppressed, drug levels can build up to potentially toxic levels. In this case, the effects can persist for extended periods of time after the consumption of grapefruit juice. As of 2013, there are at least 85 drugs shown to adversely interact with grapefruit juice (Bailey, Dresser, & Arnold, 2013). Some psychotropic drugs that are likely to interact with grapefruit juice include carbamazepine (e.g., Tegretol), prescribed for bipolar disorder; diazepam (e.g., Valium), used to treat anxiety, alcohol withdrawal, and muscle spasms; and fluvoxamine (e.g., Luvox), used to treat obsessive-compulsive disorder and depression.

Individualized therapy, metabolic differences, and potential prescribing approaches for the future
Mental illnesses contribute to more disability in Western countries than all other illnesses, including cancer and heart disease. Depression alone is predicted to be the second largest contributor to disease burden by 2020 (World Health Organization, 2004). The numbers of people affected by mental health issues are pretty astonishing, with estimates that 25% of adults experience a mental health issue in any given year, and this affects not only the individual but their friends and family. One in 17 adults experiences a serious mental illness (Kessler, Chiu, Demler, & Walters, 2005). Newer antidepressants are probably the most frequently prescribed drugs for treating mental health issues, although there is no “magic bullet” for treating depression or other conditions. Pharmacotherapy with psychological therapy may be the most beneficial treatment approach for many psychiatric conditions, but there are still many unanswered questions. For example, why does one antidepressant help one individual yet have no effect for another? Antidepressants can take four to six weeks to start improving depressive symptoms, and we don’t really understand why. Many people do not respond to the first antidepressant prescribed and may have to try different drugs before finding something that works for them. Other people just do not improve with antidepressants (Ioannidis, 2008). As we better understand why individuals differ, the easier and more rapidly we will be able to help people in distress.
One area that has received interest recently has to do with an individualized treatment approach. We now know that there are genetic differences in some of the cytochrome P450 enzymes and their ability to break down drugs. Members of the general population fall into one the following four categories: ultra-extensive metabolizers break down certain drugs (e.g., antidepressants) very quickly, extensive metabolizers are also able break down drugs fairly quickly, intermediate metabolizers break down drugs more slowly than either of the two above groups, and finally, poor metabolizers break down drugs much more slowly than all of the other groups. Now, consider someone receiving a prescription for an antidepressant; what would the consequences be if they were either an ultra-extensive metabolizer or a poor metabolizer? The ultra-extensive metabolizer would be given antidepressants and told it will probably take four to six weeks to begin working, which is true, but they metabolize the medication so quickly that it will never be effective for them. In contrast, the poor metabolizer given the same daily dose of the same antidepressant may build up such high levels in their blood because they are not breaking the drug down, that they will have a wide range of side effects and feel terrible — also not a positive outcome. What if, instead, prior to prescribing an antidepressant, the doctor could take a blood sample and determine which type of metabolizer a patient actually was? They could then make a much more informed decision about the best dose to prescribe. There are new genetic tests now available to better individualize treatment in just this way. A blood sample can determine, at least for some drugs, which category an individual fits into, but we need data to determine if this actually is effective for treating depression or other mental illnesses (Zhou, 2009). Currently, this genetic test is expensive, and not many health insurance plans cover this screen, but this may be an important component in the future of psychopharmacology.
Children and Psychopharmacology
A recent Centers for Disease Control and Prevention report (2013) has suggested that as many as one in five children between the ages of five and 17 may have some type of mental disorder (e.g., ADHD, autism, anxiety, or depression). The incidence of bipolar disorder in children and adolescents has also increased 40 times in the past decade (Moreno et al., 2007), and it is now estimated that one in 88 children have been diagnosed with an autism spectrum disorder (Centers for Disease Control and Prevention, 2011). Why has there been such an increase in these numbers? There is no single answer to this important question. Some believe that greater public awareness has contributed to increased teacher and parent referrals. Others argue that the increase stems from changes in criterion currently used for diagnosing. Still others suggest environmental factors, either prenatally or postnatally, have contributed to this upsurge.

We do not have a clearly defined answer, but the question does bring up an additional controversy related to how we should treat this population of children and adolescents. Many psychotropic drugs used for treating psychiatric disorders have been tested in adults, but few have been tested for safety or efficacy with children or adolescents. The most well-established psychotropics prescribed for children and adolescents are the psychostimulant drugs used for treating attention-deficit/hyperactivity disorder (ADHD), and there are clinical data on how effective these drugs are. However, we know far less about the safety and efficacy in young populations of the drugs typically prescribed for treating anxiety, depression, or other psychiatric disorders. The young brain continues to mature until probably well after age 20, so some scientists are concerned that drugs that alter neuronal activity in the developing brain could have significant consequences. There is an obvious need for clinical trials in children and adolescents to test the safety and effectiveness of many of these drugs, which also brings up a variety of ethical questions about who decides what children and adolescents will participate in these clinical trials, who can give consent, who receives reimbursements, and so on.
Source: Adapted from Barron (2020).
Key Takeaways
- Psychoactive drugs are commonly used in the treatment of mental disorders.
- All psychoactive drugs have side effects.
- Electroconvulsive therapy is a controversial procedure used to treat severe depression, in which electric currents are passed through the brain, deliberately triggering a brief seizure.
- A newer method of brain stimulation is transcranial magnetic stimulation, which is a noninvasive procedure that employs a pulsing magnetic coil to electrically stimulate the brain.
- Certain types of food in the stomach can alter the rate of drug absorption, and other foods can also alter the rate of drug metabolism.
- Treating children and adolescents with psychoactive drugs is controversial.
Critical Thinking Exercises
- What are some of the issues surrounding prescribing medications for children and adolescents? How might this be improved?
- What are some of the factors that can affect relapse to an addictive drug?
- How might prescribing medications for depression be improved in the future to increase the likelihood that a drug would work and minimize side effects?
Image Attributions
Figure 16.6. Used under a CC BY-NC-SA 4.0 license.
Figure 16.7. Used under a CC BY-NC-SA 4.0 license.
Figure 16.8.Used under a CC BY-NC-SA 4.0 license.
Figure 16.9. ICU IV 1 by Calleamanecer is used under a CC BY-SA 3.0 license.
Figure 16.10. Used under a CC BY-NC-SA 4.0 license.
Figure 16.11. Happy Feet by The Monkey is used under a CC BY-NC-ND 2.0 license.
References
Abrams, R. (1997). Electroconvulsive therapy (3rd ed.). Oxford, England: Oxford University Press.
Bailey, D. G., Dresser G., & Arnold J. M. (2013). Grapefruit-medication interactions: Forbidden fruit or avoidable consequences? Canadian Medical Association Journal, 185, 309–316.
Barbui, C., Esposito, E., & Cipriani, A. (2009). Selective serotonin reuptake inhibitors and risk of suicide: A systematic review of observational studies. Canadian Medical Association Journal, 180(3), 291–97.
Barron, S. (2020). Psychopharmacology. In R. Biswas-Diener & E. Diener (Eds.), Noba textbook series: Psychology. Champaign, IL: DEF. Retrieved from http://noba.to/umx6f2t8
Canadian Press. (2014). Health Canada updates safety profile of bipolar drug lithium. Canada.com. Retrieved from http://o.canada.com/health/health-canada-updates-safety-profile-of-bipolar-drug-lithium
Casey, D. E. (1996). Side effect profiles of new antipsychotic agents. Journal of Clinical Psychiatry, 57(Suppl. 11), 40–45.
Centers for Disease Control and Prevention. (2011) Prevalence of autism spectrum disorders – Autism and developmental disabilities monitoring network, 14 sites, United States, 2008. Morbidity and Mortality Weekly Report 61(SS03), 1–19.
Centers for Disease Control and Prevention. (2013) Mental health surveillance among children – United States, 2005—2011. Morbidity and Mortality Weekly Report 62(Suppl), 1–35.
Corcoran, C. D., Thomas, P., Phillips, J., & O’Keane, V. (2006). Vagus nerve stimulation in chronic treatment-resistant depression: Preliminary findings of an open-label study. The British Journal of Psychiatry, 189, 282–283.
Dougherty, D., Baer, L., Cosgrove, G., Cassem, E., Price, B., Nierenberg, A., . . . Rauch, S. L. (2002). Prospective long-term follow-up of 44 patients who received cingulotomy for treatment-refractory obsessive-compulsive disorder. American Journal of Psychiatry, 159(2), 269.
Fraser, A. R. (2000). Antidepressant choice to minimize treatment resistance. The British Journal of Psychiatry, 176, 493.
Greenhill, L. L., Halperin, J. M., & Abikof, H. (1999). Stimulant medications. Journal of the American Academy of Child & Adolescent Psychiatry, 38(5), 503–512.
Healy, D., & Whitaker, C. J. (2003). Antidepressants and suicide: Risk-benefit conundrums. Journal of Psychiatry & Neuroscience, 28, 331–339.
Hollon, S. D., Thase, M. E., & Markowitz, J. C. (2002). Treatment and prevention of depression. Psychological Science in the Public Interest, 3, 39–77.
Ioannidis, J. P. A. (2008). Effectiveness of antidepressants: An evidence myth constructed from a thousand randomized trials? Philosophy, Ethics, and Humanities in Medicine, 3(1), 14.
Kellner, C. H., Fink, M., Knapp, R., Petrides, G., Husain, M., Rummans, T., . . . Malur, C. (2005). Relief of expressed suicidal intent by ECT: A consortium for research in ECT study. The American Journal of Psychiatry, 162(5), 977–982.
Kessler, R. C., Chiu, W. T., Demler, O., & Walters, E. E. (2005). Prevalence, severity, and comorbidity of twelve-month DSM-IV disorders in the National Comorbidity Survey Replication (NCS-R). Archives of General Psychiatry, 62, 617–627.
Kowatch, R. A., Suppes, T., Carmody, T. J., Bucci, J. P., Hume, J. H., Kromelis, M., . . . Rush, A. J. (2000). Effect size of lithium, divalproex sodium, and carbamazepine in children and adolescents with bipolar disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 39, 713–20.
Loo, C. K., Schweitzer, I., & Pratt, C. (2006). Recent advances in optimizing electroconvulsive therapy. Australian and New Zealand Journal of Psychiatry, 40, 632–638.
Marangell, L. B., Silver, J. M., Goff, D. C., & Yudofsky, S. C. (2003). Psychopharmacology and electroconvulsive therapy. In R. E. Hales & S. C. Yudofsky (Eds.), The American Psychiatric Publishing textbook of clinical psychiatry (4th ed., pp. 1047–1149). Arlington, VA: American Psychiatric Publishing.
McElroy, S. L., & Keck, P. E. (2000). Pharmacologic agents for the treatment of acute bipolar mania. Biological Psychiatry, 48, 539–557.
Moreno, C., Laje, G., Blanco, C., Jiang, H., Schmidt, A. B., & Olfson, M., (2007). National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Archives of General Psychiatry, 64(9), 1032–1039.
National Institute of Mental Health. (2008). Mental health medications (NIH Publication No. 08-3929). Retrieved from http://www.nimh.nih.gov/health/publications/mental-health-medications/complete-index.shtml#pub4
Nemeroff, C., Mayberg, H., Krahl, S., McNamara, J., Frazer, A., Henry, T., . . . Brannan, S. (2006). VNS therapy in treatment-resistant depression: Clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology, 31(7), 1345–1355.
Otto, M. W., Pollack, M. H., Sachs, G. S., Reiter, S. R., Meltzer-Brody, S., & Rosenbaum, J. F. (1993). Discontinuation of benzodiazepine treatment: Efficacy of cognitive-behavioral therapy for patients with panic disorder. American Journal of Psychiatry, 150, 1485–1490.
Rado, J., Dowd, S. M., & Janicak, P. G. (2008). The emerging role of transcranial magnetic stimulation (TMS) for treatment of psychiatric disorders. Directions in Psychiatry, 28(4), 315–332.
Rohsenow, D. J., Niaura, R. S., Childress, A. R., Abrams, D. B., & Monti, P. M. (1990). Cue reactivity in addictive behaviors: Theoretical and treatment implications. International Journal of Addiction, 25, 957–993.
Sachdev, P. S., & Chen, X. (2009). Neurosurgical treatment of mood disorders: Traditional psychosurgery and the advent of deep brain stimulation. Current Opinion in Psychiatry, 22(1), 25–31.
Sackheim, H. A., Haskett, R. F., Mulsant, B. H., Thase, M. E., Mann, J. J., Pettinati, H., . . . Prudic, J. (2001). Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: A randomized controlled trial. Journal of the American Medical Association, 285, 1299–1307.
Sackheim, H. A., Prudic, J., Fuller, R., Keilp, J., Philip, W., Lavori, P. W., & Olfson, M. (2007). The cognitive effects of electroconvulsive therapy in community settings. Neuropsychopharmacology, 32, 244–254.
Simon, G. E. (2006). The antidepressant quandary: Considering suicide risk when treating adolescent depression. The New England Journal of Medicine, 355(26), 2722–2723.
Simon, G. E., Savarino, J., Operskalski, B., & Wang, P. S. (2006). Suicide risk during antidepressant treatment. American Journal of Psychiatry, 163, 41–47.
Spencer, T. J., Biederman, J., Harding, M., & O’Donnell, D. (1996). Growth deficits in ADHD children revisited: Evidence for disorder-associated growth delays? Journal of the American Academy of Child & Adolescent Psychiatry, 35(11), 1460–1469.
Spielman, R., Dumper, K., Jenkins, W., Lacombe, A., Lovett, M., & Perlmutter, M. (2019). Therapy and treatment. In OpenStax, Psychology. OpenStax CNX. Retrieved from https://cnx.org/contents/Sr8Ev5Og@10.16:quO6dhNg@9
Valenstein, E. (1986). Great and desperate cures: The rise and decline of psychosurgery and other radical treatments for mental illness. New York, NY: Basic Books.
World Health Organization. (2004). Promoting mental health: Concepts, emerging evidence, practice (Summary Report). Geneva, Switzerland: Author. Retrieved from http://www.who.int/mental_health/evidence/en/promoting_mhh.pdf
Zahn, T. P., Rapoport, J. L., & Thompson, C. L. (1980). Autonomic and behavioral effects of dextroamphetamine and placebo in normal and hyperactive prepubertal boys. Journal of Abnormal Child Psychology, 8(2), 145–160.
Zhou, S. F. (2009). Polymorphism of human cytochrome P450 2D6 and its clinical significance: Part II. Clinical Pharmacokinetics, 48, 761–804

